University of California, Berkeley | Plant & Microbial Biology
Pollen Receptor Kinases
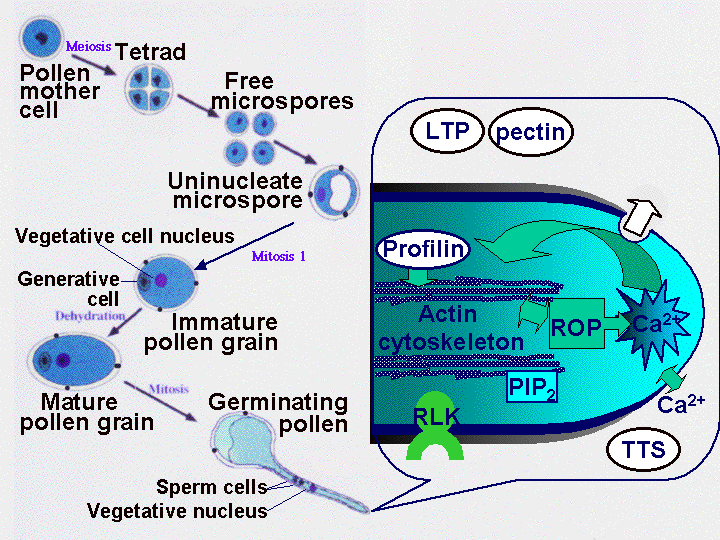
Pollen tube growth is a fascinating process. The pollen tube grows by tip growth, and the cell has to generate new plasma membrane and wall material very rapidly. A calcium gradient is important, and dynamic rearrangements of the actin cytoskeleton occur. As the pollen tube grows through the pistil, it perceives components of the style extracellular matrix (such as LTP and pectin, and TTS), as shown in the cartoon below.
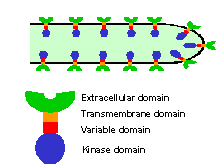
As one entrée into understanding pollen-pistil communication, we characterized three tomato receptor kinases (LePRK1, 2 and 3) that are specifically expressed in pollen. These kinases have extracellular domains composed of 5-6 leucine-rich repeats, a transmembrane domain, and other characteristic features that distinguish them from other plant receptor kinases. Because antibodies raised against the extracellular domains of the tomato kinases label the pollen tubes, it seemed likely that these kinases would mediate pollen-pistil signaling.
The Extracellular Partners
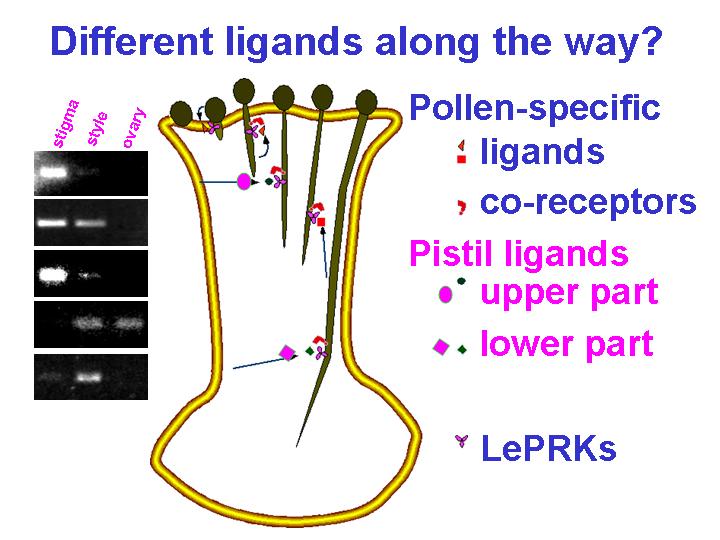
We used yeast two-hybrid screening to isolate potential ligands for these kinases. This might seem a risky approach, but indeed, in these screens (Tang et al., 2002) we found that the extracellular domains of these kinases interact with several secreted proteins that are expressed either in the pistil or in the pollen grain itself. Because some of these secreted proteins are only expressed in restricted domains of the pistil (see RT-PCR results next to cartoon, below), or are pollen-specific (Supplemental Table from Tang et al., 2002), we speculate that there may be different ligands involved in different phases of pollen tube growth. We showed that LAT52, one of the candidate ligands from pollen, can be co-immunoprecipitated with an antibody raised against the extracellular domain of LePRK2. This finding was especially exciting because we had already shown (Muschietti et al., 1994) that LAT52 plays an important role in pollen tube growth. But it was curious that the LePRK2-LAT52 interaction didn't occur after pollen started to germinate (Tang et al., 2002). We discovered a possible reason for this - it turns out that a candidate ligand from the female replaces LAT52 after pollen germinates (Tang et al., 2004). The candidate ligand from the female, LeStig1, promotes pollen tube growth. There may be more "changing of partners" - LeStig1 is not expressed near the ovary but pollen tubes still need to get there.
The Cytoplasmic Partners
We also used yeast two-hybrid screens to identify downstream signaling components. One cytoplasmic interactor (KPP - for kinase partner protein) was especially interesting. KPP is a pollen-specific peripheral membrane protein and is phosphorylated in pollen. Overexpression of KPP yields aberrant pollen tubes that are similar to the phenotypes seen when Rop or Rac (small GTPases) are overexpressed. Thus KPP may be a bridge between the receptor kinases and other cytoplasmic proteins already known to be important for polarized growth (Kaothien et al., 2005). KPP is similar to a family of 14 proteins in Arabidopsis. Nearly all were annotated as hypothetical proteins, but our RT-PCR results showed that many are expressed. Most interestingly, another group (Berken, A., Thomas, C. and Wittinghofer, A. " A new family of RhoGEFs activates the Rop molecular switch in plants", Nature June 26, 2005) showed that KPP-like proteins in Arabidopsis are Rho-GEFs. More recently we have characterized the interactions of the Arabidopsis Rho-GEFS and the Arabidopsis equivalents of the tomato pollen receptor kinases (Zhang and McCormick, 2007). This work was facilitated because we developed a robust method for in vitro pollen germination of Arabidopsis pollen (Boavida and McCormick, 2007), a technique that was previously very problematic.
